
What is hydrogen?


Hydrogen is above all the source of the universe and therefore of life. Our sun is mainly made of hydrogen, and this same hydrogen is the source of the formation of stars! Thus hydrogen is the most abundant element in the universe. And yet, in its natural state, very little is found on our planet Earth. Exploited since the 19th century, it is found in abundance in industry where it is used as a base material for the production of ammonia and methanol, and for refining petroleum products, fuels and biofuels.
Hydrogen is the simplest chemical element: it is composed of one proton and one electron. It is a very light gas whose chemical formula is H2. It is highly flammable, odorless, colorless, non-toxic and non-corrosive. The hydrogen atom is the lightest: to make the comparison, hydrogen is 14 times lighter than air! It is a very energetic gas, more than twice as much as gasoline and natural gas and three times as much as diesel!
In its natural state, it is generally combined with other atoms: it is found in water (H2O), oil (HC hydrocarbons) and natural gas (CH4 composition). Certain techniques and chemical processes such as thermochemical or photochemical decomposition, steam reforming, biomass pyrolysis or water electrolysis allow hydrogen to be separated from the elements with which it is associated.
Let’s specify that making hydrogen in a conventional way, i.e. by using steam reforming of natural gas, is not very ecological. This technique has the disadvantage of releasing large quantities of greenhouse gases: the manufacture of 1 kg of hydrogen by steam reforming produces… 10 kg of CO2! The solution is therefore to manufacture hydrogen from the electrolysis of water in plants powered by renewable energies, a technology called “Power to gas”. Fortunately, this technology is currently favored by cities and regions that are installing hydrogen production stations all over France.
The world consumption of hydrogen today is 74 million tons, representing less than 2% of the world energy consumption.
Hydrogen is a gas whose chemical properties offer a major energy interest. It is considered as an “energy carrier” because it can be stored, transported and used after being produced. The energy contained in hydrogen can be recovered in two ways: by burning it or through a fuel cell. It is through these fuel cells that hydrogen as an energy carrier offers a wide range of uses, from energy storage to the supply of electricity for buildings or the powering of vehicles.
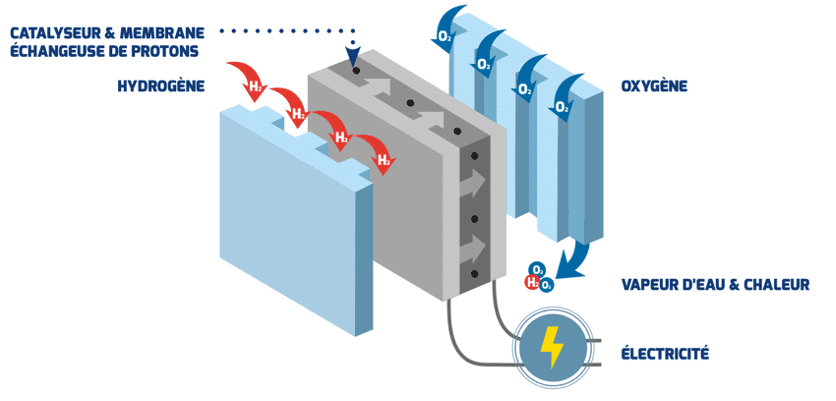
A short electrochemistry lesson: a fuel cell is supplied on one side with dihydrogen (H2, commonly called “hydrogen”) and on the other with dioxygen (O2). The hydrogen passes through a proton exchange polymer membrane, releasing electrons that will ensure the production of electricity. The protons are recovered and the final reaction releases only water while also producing heat (which can be reused to heat buildings or even vehicles).
In a world facing ever more pressing environmental issues, transition
“A stone has no hope of being anything other than
The fourth leading cause of mortality in the world, air